PROJECT OVERVIEW
Context

The COVID-19 pandemic, caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), has undeniably emerged as one of the largest global health threats to humanity this century. The rapid rate of its global spread and observed mortality raises public health, socio-economic and scientific challenges at an unprecedented scale, well beyond those of the previous coronavirus outbreaks [SARS in 2003 and MERS (Middle East Respiratory Syndrome) in 2012].
The wide spectrum of clinical symptoms, disease severity in high-risk individuals, transmission efficiency and mortality raises immediate challenges for research as well as patient care. This has made it imperative for the scientific community to immediately collaborate under public-private partnerships to identify efficacious and safe drugs. More than ever before, collaborative basis and applied research are pivotal to creating a foundation for rapid responses to the ongoing crisis, until vaccines counter the pandemic through the immunization of the global population, and for long-term preparedness, in case of unexpected future outbreaks.
Objectives & Strategy

The CARE consortium has three main goals:
- The development of therapeutics to provide an emergency response towards the current COVID-19 pandemic
- The development of therapeutics to address the current and/or future coronavirus outbreaks
- The understanding of the physiopathology of COVID-19 and the discovery of immune markers contributing to the host immune responses to COVID-19 infection and their correlations with clinical and virological outcomes
The project ambition is to accelerate development and use of effective therapies to COVID-19 patients in Europe and around the world, and generate a truly transformative treatment regimen. The project combines a focus on rapid emergency response with focus on long-term preparedness for future outbreaks.
1
EMERGENCY RESPONSE
The development of therapeutics to provide an emergency response towards the current COVID-19 pandemic.

Drug positioning / repurposing
Screening and profiling compound libraries to progress molecules to advanced stages of clinical testing within 18 months.
2
LONG TERM STRATEGY
The development of therapeutics to address the current and/or future coronavirus outbreaks.

Small molecule drug discovery
Target-based and in silico screening and profiling of candidate compounds against SAS-CoV-2 for clinical testing in 36 months.

Virus-neutralising antibody discovery
Fully human phage and yeast display, immunisation of humanised models, patient B cells and in silico design for clinical testing within 36 months.
3
INVESTIGATING COVID-19
Increasing the understanding of the physiopathology of COVID-19.

Immune marker discovery
Discovery of immune markers contributing to host-immune responses to COVID-19 infection and their correlations with clinical and virological outcomes.
The overarching vision of the CARE Consortium is to create effective therapies for current and future coronaviral outbreaks, which have a high safety profile.
In accordance, the CARE Consortium proposes five main research objectives:
1
To identify therapeutic candidates against the current SARS-CoV-2, other potentially emerging SARS-CoV-2 clades, and related coronavirus genera, from repurposed drugs
2
To identify novel therapeutic candidates against the current SARS-CoV-2, other potentially emerging SARSCoV-2 clades, and related coronavirus genera
3
To identify immune markers contributing to the host immune responses to COVID-19 infection and their correlations with clinical and virological outcomes
4
To pre-clinically assess ADME, PK/PD, potency, and safety of therapeutic candidates in vitro and in animal models
5
To advance lead candidates into Phase 1 and Phase 2 clinical trials in humans
How will the objectives regarding new treatments be achieved?
To address the research objectives, the CARE consortium carefully designed an integrated research programme centered around three main pillars:
- Drug repositioning, by screening and profiling compound libraries contributed by partners with the aim of rapidly progressing molecules to advanced stages of clinical testing
- Small-molecule drug discovery based on in silico screening and profiling of candidate compounds directed against SARS-CoV-2 and future coronavirus targets
- Virus neutralizing antibody discovery using fully human phage and yeast display, immunisation of humanised animal models, patient B cells and in silico design
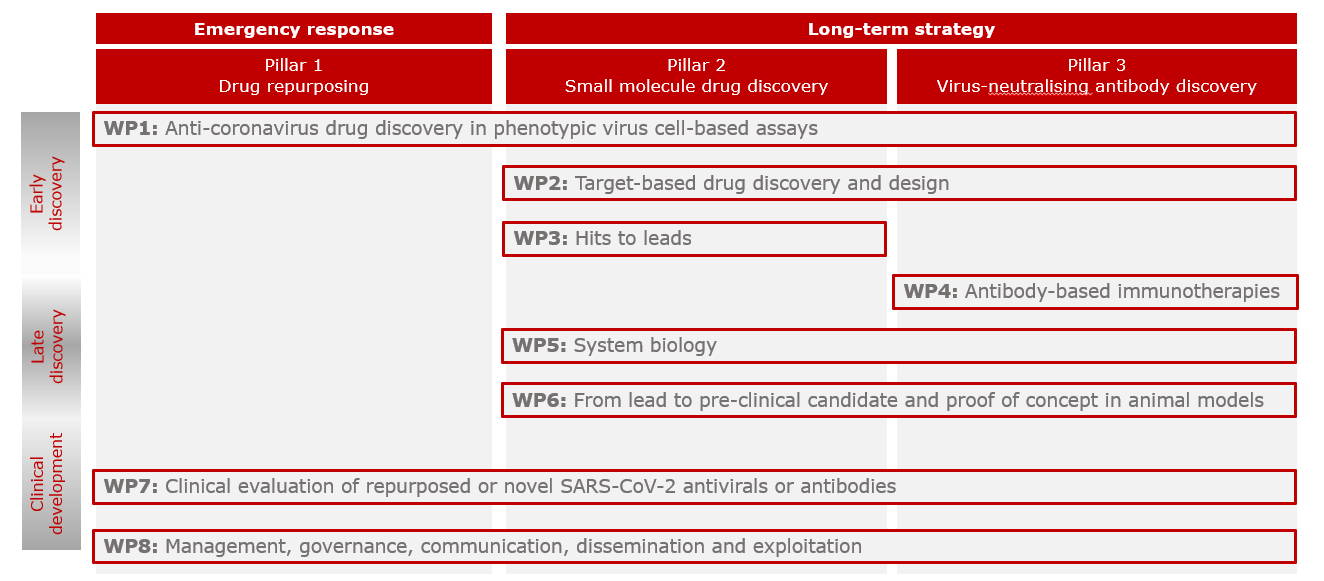
The CARE consortium set up eight interrelated work-packages (WPs) to carry out the project activities and achieve the five main outcomes.
- WP1 Anti-coronavirus drug discovery in phenotypic virus-cell-based assays: identify small molecule therapeutic candidates against the current SARS-CoV-2 and potentially other emerging clades and related coronaviruses in phenotypic virus-cell based assays, through screening of repurposed drug libraries, small molecule libraries, or biologicals
- WP2 Target-based drug discovery and design: identify novel small molecule therapeutic candidates against the current SARS-CoV-2 by target-based drug-design
- WP3 Hits to leads: generate and optimize small molecule leads towards drug candidates for preclinical and clinical evaluation
- WP4 Generation and characterisation of monoclonal antibodies against SARS-CoV-2 and related coronaviruses: identify neutralising monoclonal antibodies against SARS-CoV-2
- WP5 System biology: identify immune markers contributing to the host immune responses to SARS-CoV-2 infection and their correlations with clinical and virological outcomes
- WP6 From lead to pre-clinical candidate and proof-of-concept in in vivo models: pre-clinically assess ADME, PK/PD, potency and safety of therapeutic candidates in vitro and in vivo models
- WP7 Clinical evaluation of repurposed or novel SARS-CoV-2 antivirals or antibodies: advance lead candidates into Phase I and Phase 2 clinical trials in humans
- WP8 Management, ethics, communication, dissemination and exploitation: efficiently manage CARE activities across partners and sectors, ensuring the highest standards of compliance to and effective strategic interactions with ethical and regulatory authorities
Expected Results

Our ambition is to enable the discovery and development of novel medicines and a sustainable scientific research and evidence-based environment in Europe, spanning across disciplines and sectors, to rapidly respond to current and future global coronavirus outbreaks.
Our ambition is:
- to enable the research and clinical community to quickly advance effective therapies to COVID-19 patients in Europe
- to advance in-depth functional and structural understanding of target-lead and virus-host interactions to further support the rational and AI-based identification and design of therapeutic leads against SARS-CoV-2 and other species and genera of coronaviruses
- to open new areas of biology-informed drug discovery and seed a massive, inclusive community-driven effort for the development of other drugs (against other coronavirus targets)
- to implement an integrative and cross-sectorial research and development program that fosters synergies between research, industry and the clinic towards the development of effective therapies and improved evidence-based patient management
- to contribute to the advancement of coronaviral science and also stimulate economic growth through collaborating SMEs
- to provide long-term, but also tangible short-term health benefits to Europe
- to provide innovative medicines and enabling resources for target identification and validation as well as to encourage community collaboration
- to create sustainable knowledge and tools,
- to train the next generation of biomedical and antiviral researchers
- to proactively incorporate the aspects of coronavirus variants into the project’s strategy