October 24th 2024
A picture paints a thousand words: Utrecht University expertise in Cryo-EM technology continues to bring benefit to CARE’s small molecule and antibody development work
Dr. Daniel Hurdiss, Assistant Professor, Utrecht University
Cryogenic electron microscopy (cryo-EM) capability has been made available to CARE through the expertise of CARE partner, Utrecht University (UU). Virologists from UU’s Biomolecular Health Sciences department use cryo-EM as one of their core techniques to study viruses, under the leadership of Dr. Daniel Hurdiss who has used this method to study virus structures over the last 10 years.
Cryo-EM, or cryogenic electron microscopy, is a powerful imaging technique used to study the structures of biological molecules at high resolution. It involves flash-freezing samples in vitreous ice to preserve their natural state, followed by imaging with an electron microscope, and subsequent computational analysis to determine a three-dimensional structure.
Why Cryo-EM is so important for CARE’s research
The importance of cryo-EM for coronavirus pandemic preparedness and responsiveness cannot be overstated. The first human coronavirus spike structure was obtained using this technique in 2016, and this served as a roadmap for the development of pre-fusion stabilized vaccines. Similarly, this technique was essential to visualize other coronavirus proteins for the first time, such as the RNA-dependent RNA polymerase (RdRp) complex and membrane protein. Within CARE, all these proteins, and more, have been investigated as targets for pharmacological intervention. As such, cryo-EM plays an essential role in understanding the molecular mechanism of candidate antiviral molecules, as well as facilitating their optimization.
Cryo-EM and small molecule development in CARE
In Work Package 2 (Target-based drug discovery and design), cryo-EM was used to study how a macrocyclic peptide inhibitor, developed by UU, binds to the SARS-CoV-2 spike protein. This three-dimensional information is now being used to guide the rational improvement of this molecule.
Cryo-EM was also essential for determining the mechanism of action of a first-in-class SARS-CoV-2 membrane protein-targeting compound, developed by CARE partner KU Leuven. This data can now be used to further improve the potency and breath of these molecules.
In the same vein, Cryo-EM was key in determining the mechanism of action of Bemnofosbuvir, a nucleotide analogue prodrug initially developed against the Hepatitis C virus and repositioned in 2020 against SARS-CoV-2. The drug binds to two independent sites in nsp12, namely the RNA dependent RNA polymerase and the NiRAN domain, opening avenues to further improve the design of novel inhibitors.


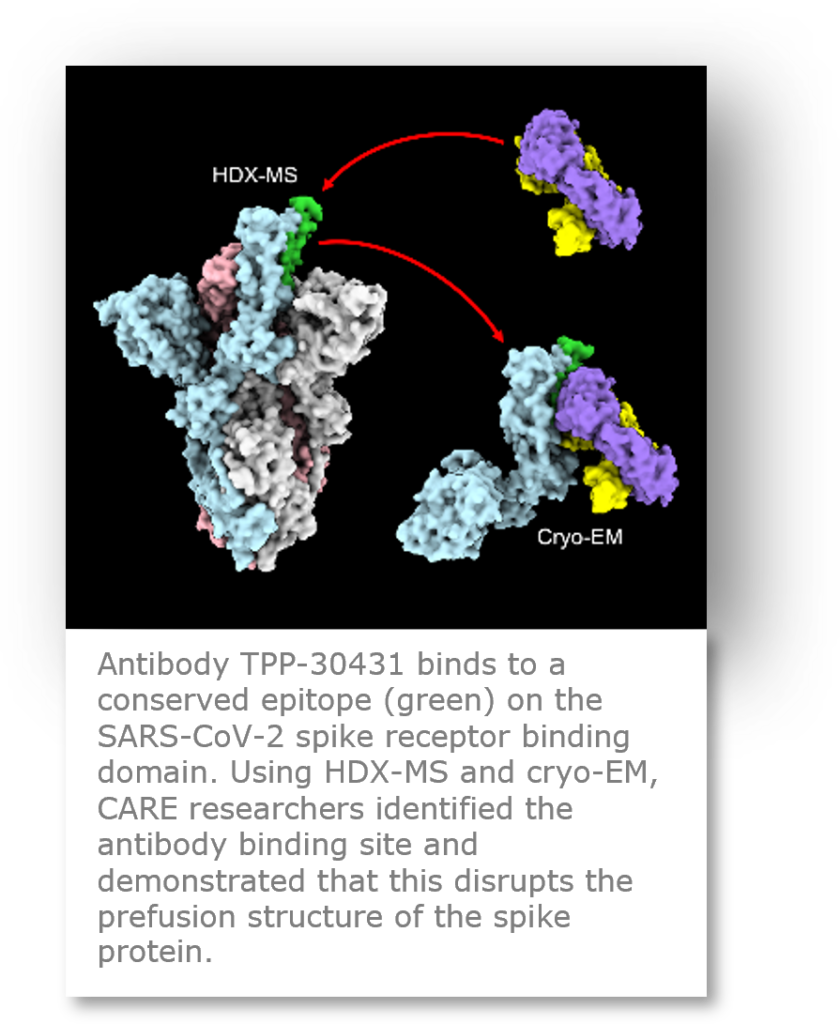
Cryo-EM and antibody development in CARE
Cryo-EM structural analysis is a powerful tool for binding epitope mapping of antibodies targeting the viral spike protein and inhibiting SARS-CoV-2 cell entry. In Work Package 4 (Antibody development), many cryo-EM structures of spike-antibody complexes were determined by UU and CHUV. Defining the different epitopes of neutralizing antibodies allows us to understand their mechanism of action and to increase our knowledge of how antibodies can confer protective immunity against SARS-CoV-2 infection. Furthermore, precise knowledge of an antibody’s epitope allows a scientist to select antibody combinations that effectively target the viral spike protein without the antibodies interfering with each other, and to select antibodies that bind to more conserved regions of the spike that are less susceptible to mutations that could confer viral resistance to the neutralizing antibodies. Computational antibody design is another emerging field that requires structural data to generate therapeutic antibodies with improved potency and neutralizing breadth against the emerging variants of concern.
How might it be used in the future?
Given how important the knowledge derived from structural biology has been prior to, and during, the COVID-19 pandemic, it is safe to assume that this method will continue to be used to provide fundamental insights into the structure and function of viral proteins and facilitate the development of vaccines and antiviral molecules. Conceivably, with the ongoing improvements in AI for small molecule and antibody design, these methods can be used synergistically to design and validate future antiviral therapies. Thinking even further ahead, developments in cryo-EM which allow viral proteins to be visualised inside the infected cell may reveal new druggable targets that can be exploited in the future.
What added value has Cryo-EM brought to CARE?
Beyond the valuable biological insights cryo-EM provides for drug discovery efforts within CARE, these data also provide a wonderful instrument for communicating scientific results and concepts to a wider audience. After all, a picture paints a thousand words.
